Medical Device Recall Information
Philips Respironics Sleep and Respiratory Care devices
Philips Respironics Sleep and Respiratory Care devices
If you haven't yet registered your device
Home > Replacement device safety information
Replacement device safety information
August 5, 2022
While we work to provide replacement devices as quickly as possible, we want to share the steps we are taking to ensure your replacement device is safe to use so you can be confident in your new device.

While affected devices contained a polyester-based polyurethane (PE-PUR) sound abatement foam component, the sound abatement foam in new and recertified CPAP, BiPAP and Trilogy devices is a silicone foam that has met all applicable industry testing standards, including particulate and Volatile Organic Compounds (VOCs) emissions testing.
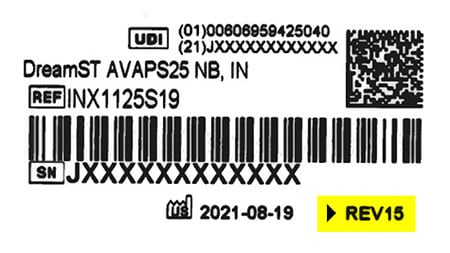
All remediated devices will include a label at the bottom showing REV15 or higher. This indicates that the device has been remediated to include silicone foam and is safe to use.

Regular device cleaning and maintenance is important for your therapy

Need further assistance?
Talk to an agent by calling one of our international call center lines.
