Let's talk
Learn more about hospital respiratory care solutions including high-flow oxygen therapy (HFT), noninvasive ventilation (NIV) and invasive mechanical ventilation (IMV).
Fill out the form below to get started.
Philips Optimal NIV pairs the right technology with the right training to help ensure success across the spectrum of care. The respiratory technology - the devices, circuits and patient interfaces - offers the flexibility needed for advanced patient care. The training - knowing what technique to use, how to use it and when it may be appropriate - offers knowledge that not only helps enhance patient care but also can benefit the bottom line in a big way.
Rethink Respiratory Care
Patient condition dictates treatment, but sometimes more than one technique may be appropriate. When multiple techniques are viable, more frequent use of NIV – with or without complementary HFT – can help reduce the higher costs of IMV. More importantly, managing NIV can help improve patient care.
 Optimal NIV reduced hospital length of stay by more than
Optimal NIV reduced hospital length of stay by more than3days¹
 Optimal NIV resulted in a
Optimal NIV resulted in a16%
redution in mortality² Optimal NIV resulted in a
Optimal NIV resulted in a38%
redution of endotracheal intubation² Hypercapnic patients placed on NIV with HFT reduced risk of reintubation vs HFT alone by
Hypercapnic patients placed on NIV with HFT reduced risk of reintubation vs HFT alone by62%³

Improve the experience
Philips supports clinicians in technique training, streamlines workflow and offers cutting-edge technology to help improve patient care.

Enhance patient care
Knowing when to use HFT or NIV instead of IMV - and how tomanage NIV - can help enhance patient care.

Manage your costs
Using IMV or failing on NIV has significant financial impacts. Managing NIV and increasing use benefit the bottom line.
Optimal NIV with Philips
Philips hospital respiratory care solutions can help continually evaluate each patient's response to treatment so you can make fast, smooth transitions.
-
V60 Plus
Philips V60 Plus* ventilator is a microprocessor-controlled, bilevel positive airway pressure (BiPAP) ventilatory assist system that provides noninvasive positive pressure ventilation (NPPV) and invasive ventilatory support for spontaneously breathing adult and pediatric patients.
850008 -
Trilogy EV300
Transition to the future today with the next generation of Philips ventilators. The Trilogy EV300 ventilator delivers enhanced performance in noninvasive (NIV) and invasive (IV) ventilation, so patients can be treated with a single device throughout their hospital stay, regardless of changing conditions. Trilogy EV300 is designed to stay with your patients, saving your staff time and effort as patients move between hospital departments.
DS2200X11B -
Respironics V60
The Respironics V60 ventilator is a microprocessor-controlled, bilevel positive airway pressure (BiPAP) ventilatory assist system that provides noninvasive positive pressure ventilation (NPPV) and invasive ventilatory support for spontaneously breathing adult and pediatric patients.
989805611761 -
Respironics PN841
The PN841 pediatric nasal mask is truly designed for children, with a modified cushion curvature for the specific sizing and bone structure of pediatric patients. This small and light mask comes in a range of cushion sizes, features a convenient leak correction dial, and has a child-friendly fabric pattern to provide a positive experience for even your smallest patients.
1126611 -
AC611
The Philips High Flow Nasal Cannula AC611 provides comfortable, stable oxygen delivery, and employs the same circuit as our NIV masks to help you control costs and enhance workflow. It is compatible with our V60 Plus ventilator to support the use of noninvasive ventilation (NIV) and high flow therapy (HFT) with a single system.
989805656871 -
Respironics AF531
The Respironics AF531 mask offers features that address patient comfort, proper mask fitting and ventilator compatibility.
NOCTN102 -
Respironics AF541
Designed to deliver high-quality noninvasive ventilation while resting comfortably on the face, Philips Respironics AF541 NIV mask features interchangeable under-the-nose and over-the-nose cushions to achieve the benefits of mask rotation while using a single mask.
1120925
Transitioning between respiratory techniques
Clinicians can manage patients in respiratory failure using a range of respiratory techniques – usually based on the need for oxygenation support alone or the need for both oxygenation and ventilation support. HFT provides a high level of oxygenation but only limited and somewhate variable ventilation support. NIV provides both oxygenation and entilation support. Other factors, such as the patient interface and device performance, can be consideratinos in determining when to use a particular modality once oxygenation and ventilatory needs are addressed. Click the respiratory technique below to align technique with patient severity
Click the respiratory technique below to align with patient severity.
Timely transition of therapies is important - whether escalation or de-escalation of therapy - or providing HFT between NIV sessions. Although the ability to escalate therapy is important, preventing the delay of intubation in patients who need invasive mechanical ventilation requires understanding and recognition of predictors of failure.
Clinical insights
Are HFOT and NIV complementary for acute respiratory failure?
FJ Belda, MD, PhD
Providing evidence- based care to patients in need of respiratory support
T Piraino, RRT, FCSRT
Evidence-based practice for noninvasive ventilation and high flow nasal cannula
T Piraino, RRT, FCSRT
Webinar: High flow nasal cannula and non-invasive ventilation
T Piraino, RRT, FCSRT
Related content

Succeed in Respiratory Care: NIV and High-Flow Oxygen Therapy
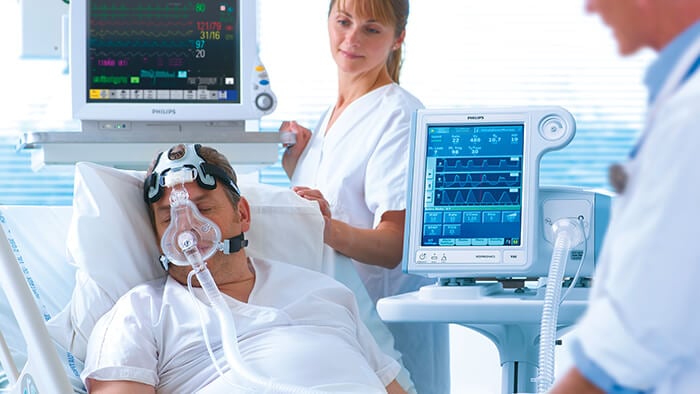
Philips Respironics V60 Hospital Ventilator

Hospital Respiratory Ventilation Solutions
References: 1. Hamadziripi N, Gale N, Hopkinson JB. Experiences of noninvasive ventilation in adults with hypercapnic respiratory failure: a review of evidence.Eur Respir Rev. 2016;25(142):451-471. doi:10.1183/16000617.0002-2016 2. Lightowler JV, Wedzicha JA, Elliott MW, Ram FSF. Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. BMJ. 2003;326(7382):185. doi:10.1136/bmj.326.7382.185 3. Thille AW, Muller F, Gacouin A, et al. Effect of postextubaton high-flow nasal oxygen with noninvasive ventilation vs high-flow nasal oxygen alone on reintubation among patients at high risk of extubation failure: a randomized clinical trial. JAMA. 2019;322(15):1465-1475. doi:10.1001/jama.2019.14901 4. Costs for NIV derived from nThrive healthcare database 2018 (https://www.nthrive.com/analytics/). 5. Software version 3.00 is a modification to the existing V60 ventilator and is therefore provided for use in accordance with FDA’s recent guidance, Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency, Section IV Policy for Modifications to FDA-Cleared Devices, issued March 2020. It is intended to remain in effect only for the duration of the public health emergency related to COVID-19 declared by the Department of Health and Human Services (HHS) 6. Drake. High-Flow Nasal Cannula Oxygen in Adult: An Evidence-based Assessment. Ann Am Thoracic Society. 2018;15(2): 145-155. 7. Schmidt, Pellegrino, Combes, Scheinkestel, Cooper, Hodgson. Mechanical ventilation during extracorporeal membrane oxygenation. Critical Care. 2014;18:203.